Abstract
Background: Poor- and intermediate-risk AML patients (pts) who are not candidates for allo-SCT have high risk of disease relapse. Data from pre-clinical studies have suggested potential roles for PD-1/PD-L1 inhibitors in AML. We hypothesized that immunomodulation with checkpoint inhibitors could stimulate anti-leukemia immune response and prevent or delay disease relapse.
Methods: We conducted a multi-center, open-label, randomized phase II study through NCI CTEP and ETCTN to assess the efficacy of nivolumab (Nivo) as maintenance therapy for pts with AML in first complete remission (CR) or CR with incomplete hematologic recovery (CRi) who were not candidates for SCT (ClinicalTrials.gov Identifier: NCT02275533). Pts were enrolled within 60 days after bone marrow biopsy confirming after recovery from final chemotherapy. Pts were stratified and randomized to observation (Obs) or Nivo (3mg/kg IV every 2 weeks for forty-six doses). The primary endpoint was progression-free survival (PFS) defined as time to disease relapse or death due to any reason. We hypothesized that Nivo maintenance would improve the 2-year PFS rate from 25% to 45% compared to Obs. To detect an effect of this magnitude (hazard ratio of 0.58), a sample size of 80 pts (40 per arm) and 62 events were required to provide 80% power at a one-sided alpha level of 0.10. Secondary endpoints included overall survival (OS), and evaluation of adverse events following Nivo administration.
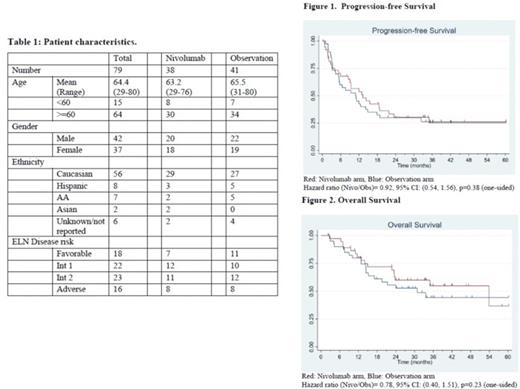
Results: Eighty pts were enrolled from October 2015 through November 2019 at 26 sites across the USA; 79 pts were deemed evaluable. Data cutoff was 6/21/2022. The characteristics of the pts randomized to Nivo or Obs arm were comparable (Table 1).
With median duration of follow-up of 24 months (33 months among survivors), 55 events (disease relapse or death) have been observed. The median number of treatment cycles in the Nivo group was 16 (range 2-46). Twenty-six pts (68%) on the Nivo arm (3 died without prior relapse) compared to 29 pts on the Obs arm (71%) have relapsed or died (1 died without prior relapse). Median PFS was 13.2 months in the Nivo arm (95% CI: 8.5-21.8) and 10.9 months in the Obs arm (5.4-14.9 months). The 2-year PFS was 30.3% in the Nivo arm (16.2-45.8%) and 30% in the Obs arm (16.8-44.4%) (Figure 1). Cox regression model generated hazard ratio (HR) (Nivo/Obs)= 0.92; 95% CI: 0.54, 1.56; p=0.38 (one-sided). For OS, 22 pts on the Nivo arm (58%) are still alive compared to 21 pts on the Obs arm (51%). The median OS was 53.9 months in the Nivo arm and 30.9 months in the Obs arm. The 2-year OS was 60.0% in the Nivo arm (41.9-74.1%) and 52.8% in the Obs arm (35.9-67.2%). Cox regression model generated HR (Nivo/Obs)= 0.78; 95% CI: 0.40, 1.51; p=0.23 (one-sided). There were more AEs of any type (regardless of attribution) on the Nivo arm; 27 (71.0%) pts on the Nivo arm had a grade 3 or higher AE compared to five pts (12.2%) on the Obs arm (p<0.001). Eight pts on the Nivo arm had a SAE compared to 3 pts on the Obs arm (p=0.11). The AEs on the Nivo arm were all expected and manageable; the most common AEs on the Nivo arm were fatigue (42%); anemia, hypertension and AST elevation (37%); diarrhea (34%) and hyperglycemia (32%).
Conclusions: Nivolumab maintenance after AML chemotherapy failed to improve the PFS and OS in this randomized Phase II study. There were increased AEs and SAEs with nivolumab maintenance, but these AEs and SAEs were expected and manageable. Correlative studies might help to identify AML pts who could benefit from nivolumab maintenance.
Disclosures
Liu:Nkarta: Honoraria, Other: Advisory board meeting ; CTI Biopharm: Honoraria, Other: Advisory board meeting; Pfizer: Honoraria, Other: Advisory board meeting and lecture speaker, Speakers Bureau; Agios: Honoraria, Other: Advisory board meeting; NGM Biopharma: Consultancy; Servier: Honoraria, Other: Advisory board meeting. Sweet:Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AROG: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Syntrix Pharmaceuticals: Research Funding; berGenBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mablytics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Yaghmour:USC: Current Employment. Jonas:Genentech: Consultancy, Research Funding; 47: Research Funding; BMS: Consultancy, Research Funding; Gilead: Consultancy, Other: data monitoring committee , Research Funding; GlycoMimetics: Consultancy, Other: protocol steering committee , Research Funding; AbbVie: Consultancy, Other: Travel Reimbursement, Research Funding; Jazz: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Servier: Consultancy; Takeda: Consultancy; Tolero: Consultancy; Treadwell: Consultancy; Accelerated Medical Diagnostics: Research Funding; Amgen: Research Funding; AROG: Research Funding; BMS: Consultancy, Research Funding; Celgene: Research Funding; Daiichi Sankyo: Research Funding; F. Hoffmann-La Roche: Research Funding; Forma: Research Funding; Roche: Research Funding; Hanmi: Research Funding; Immune-Onc: Research Funding; Incyte: Research Funding; Loxo Oncology: Research Funding; LP Therapeutics: Research Funding; Pharmacyclics: Research Funding; Sigma Tau: Research Funding. Schimmer:Otsuka: Consultancy; Jazz: Consultancy; Takeda: Consultancy, Research Funding; Novartis: Consultancy; Medivir: Research Funding; BMS: Consultancy, Honoraria, Research Funding; Astra-Zeneca: Consultancy, Honoraria. Zeidan:Astex, Medimmune, Astrazeneca, ADC Therapeutics: Research Funding; Celgene/BMS, AbbVie, Pfizer, Boeringer-Ingelheim, Trovagene, Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, Amgen, Otsuka: Consultancy, Honoraria, Research Funding; Novartis, Cardiff Oncology, Pfizer: Other: Travel Support; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie: Consultancy, Honoraria, Other: Advisory Board; Celgene/BMS, Novartis, AbbVie, Gilead, Kura, Loxo Oncology, Geron: Other: Clinical Trial Committee; Gilead, Kura, Loxo Oncology: Consultancy, Honoraria, Other: Clinical Trial Committee; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie, Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Astex, Pfizer, Medimmune/AstraZeneca, ADC Therapeutics: Research Funding; Jazz, Agios, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, Beyondspring, Gilead, Kura, Tyme, Janssen, Syndax, Geron, Ionis, Epizyme: Consultancy, Honoraria; Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Gilead, Kura, Loxo Oncology, Otsuka, Jazz, Agios, Acceleron, Astellas, Daiichi-Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Ionis, Epizyme, Janssen, Syndax, Genentec: Consultancy, Honoraria, Other: Advisory Boards. Hildebrandt:Incyte: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; RAPA Therapeutics: Membership on an entity's Board of Directors or advisory committees; Jannsen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Moderna: Current equity holder in private company; Caretrust Reit Inc: Current holder of stock options in a privately-held company; Medical PPTYS: Current holder of stock options in a privately-held company; Aimmune Therapeutics: Current holder of stock options in a privately-held company; Charlottes Webb: Current equity holder in private company; Pfizer: Current equity holder in private company; Bluebird Bio: Current equity holder in private company; CVS Health: Current equity holder in private company; Cellectis: Current holder of stock options in a privately-held company; Clovis Oncology: Current equity holder in private company; Cardinal Health: Current equity holder in private company; GW Pharmaceutical: Current equity holder in private company; Axim Biotechnologies: Current holder of stock options in a privately-held company. Hourigan:TwinStrand Biosciences: Other; Qiagen: Other; Mission Bio: Other; Archer Diagnostics: Other; Sellas Life Sciences (Inst): Research Funding. Palmisiano:Genentech: Research Funding; AbbVie: Research Funding. Salhotra:BMS: Research Funding; Orca Bio: Research Funding; Kadmon: Other: Advisory board meeting . Tzachanis:BMS: Consultancy, Research Funding; EUSA: Consultancy; Partner: Consultancy; Takeda: Consultancy. Baer:Takeda: Research Funding; Kite, a Gilead Company: Research Funding; Ascentage: Research Funding; Kura Oncology: Research Funding; AbbVie: Research Funding; Forma: Research Funding. Lin:AbbVie, Aptevo, Astellas Pharma, Bio-Path Holdings, Celgene, Celyad, Genentech-Roche, Gilead Sciences, Incyte, Jazz Pharmaceuticals, Mateon Therapeutics, Ono Pharmaceutical, Pfizer, Prescient Therapeutics, Seattle Genetics, Tolero, Trovagene: Research Funding. Patel:Agios: Membership on an entity's Board of Directors or advisory committees. Stadler:Apotex, DRL, Mylan, Sandoz: Consultancy; Calico Life Sciences, Caremark/CVS, EMA Wellness: Consultancy; CME providers (sponsorship unknown): Applied Clinical Education, Dava Oncology, Global Academy for Medical Education, OncLive, PeerView, Research to Practice, Vindico: Speakers Bureau; Astra-Zeneca, Bayer, Merck, Pfizer, Treadwell Therapeutics: Other: DSMB. Odenike:Abbvie; Impact Biomedicines; Celgene; Novartis; BMS; Taiho Pharmaceutical; CTI; Threadwell therapeutics; Bristol-Myers Squibb/Celgene (Inst): Consultancy; Celgene (Inst); Incyte (Inst); Astex Pharmaceuticals (Inst); NS Pharma (Inst); Abbvie (Inst); Janssen Oncology (Inst); OncoTherapy Science (Inst); Agios (Inst); AstraZeneca (Inst); CTI BioPharma Corp (Inst); Kartos Therapeutics (Inst); Aprea AB (Inst): Research Funding. Larson:Servier: Consultancy; Novartis: Consultancy, Research Funding; MorphoSys: Consultancy; MedPace: Consultancy; CVS/caremark: Consultancy; BMS: Consultancy; Immunogen: Consultancy; Astellas: Consultancy, Research Funding; Takeda: Consultancy; Epizyme: Consultancy; Amgen: Consultancy; Daiichi Sankyo: Research Funding; Rafael Pharmaceuticals: Research Funding; Gilead: Research Funding; Celgene: Research Funding; cellectis: Research Funding. Gajewski:Merck, Jounce, Fog Pharma, Adaptimmune, Pyxis, Allogene, Catalym, Bicara, Maia, Samyang: Consultancy, Other: Advisory board meeting ; BMS, Merck, Seattle Genetics, Evelo, Bayer, Pyxis: Research Funding; Jounce, Pyxis: Other: Cofounder/shareholder; Aduro, Evelo, BMS: Other: Intellectual property/licensing. Stock:MorphoSys: Honoraria; Kura Oncology: Honoraria; Servier: Honoraria; Pluristem: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Kite: Honoraria; Jazz Pharmaceuticals: Honoraria; Syndax: Consultancy, Honoraria; Newave Pharmaceuticals: Consultancy; Amgen: Honoraria; Agios: Honoraria.
OffLabel Disclosure:
Nivolumab
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal